Experts in Geneva dive deep into emerging viruses and new vaccines – Part 1
A symposium at Campus Biotech in Geneva, Switzerland, brought together hundreds of participants and dozens of speakers to present the latest advances in research to "prevent the next pandemic."

From April 7th-9th, 2025, the University of Geneva (UNIGE) Faculty of Medicine, Hôpitaux Universitaires de Genève (HUG) and canton of Geneva, hosted a 2.5 day event bringing together scientists, physicians and industry experts worldwide. The purpose was to present work on tracking emerging viruses and the development of new vaccines in an effort to prevent the next pandemic and “save millions of lives.”
This joint symposium, organised by the Geneva Centre for Emerging Viral Diseases (part of the WHO Global Outbreak Alert and Response Network) and the Centre for Vaccinology, was sponsored by CEPI, Sanofi, Moderna, GSK, Bavarian Nordic and Roche, among others. The registration fee for attendance in person as a regular participant was CHF450/US$520, with industry members charged CHF600/US$700.
Symposium participation was awarded 18 continuing education credits by the Swiss Society of Infectiology (SSI) and 18 continuing education credits by the Swiss Society of Tropical and Travel Medicine (SSTTM).
Inside an auditorium filled with ~200 people from all over the world and dozens participating via Zoom, this event was a melting pot of brains boiling hot in the pursuit of the next pandemic virus.
The packed program of international speakers—including Maria Van Kerkhove PhD (WHO), Marion Koopmans (Erasmus MC), Dr. Jacob Cramer (CEPI), Melanie Saville (PATH) and many, many more—showcased the latest developments in vaccines targeting viral organisms from Marburg to West Nile virus, Ebola to Nipah virus, influenza to dengue, Oropouche to Zika, and Mpox to poliomyelitis.
From mosquitos to monkeys, from bats to birds, many potential vectors of infectious disease and spillover events were identified as being high-risk in the current pandemic prevention landscape.
Opening the symposium was Maria Van Kerkhove PhD, WHO’s Acting Director of the Department of Epidemic and Pandemic Threat Management. Recovering from a recent respiratory infection, she took the stage and launched into an impassioned presentation on the importance of “Mitigating the risk of emerging viruses: WHO’s Health Emergencies Programme.”
Highlighting that “health of all peoples is fundamental to the attainment of peace and security,” she outlined the efforts of the WHO, its member states and non-state actors to ensure better pandemic preparedness and equity through a One Health approach—which would be a recurrent buzzword throughout the symposium.
Dr. Van Kerkhove addressed the importance of reaching an accord through the Pandemic Treaty—since agreed on May 20, 2025 at the 78th World Health Assembly. Remember it is not “if” but “when” there is the next pandemic, so “we cannot afford to be complacent” she stated, while asserting that the WHO’s mission is to “protect 7 billion people” from pandemic threats.
External threats are identified as:
Conflict & insecurity
Climate change and environment degradation
Zoonotic spill over events
Human migration & displacement
Demographic shifts
Geopolitical change
Evolving science & technology
Threats currently unknown
Let’s sidestep her presentation for just a moment…
One of the proposed solutions to the threats identified may well just amplify the problem.
Enter the WHO Pathogen Access and Benefit-Sharing (PABS) system—under Article 12 of the Pandemic Treaty—a “proposed mechanism aimed at facilitating the rapid and systematic sharing of biological materials and related information during outbreaks of emerging diseases.”
This system is intended to apply to all pathogens with pandemic potential, including information on their genomic sequences, and is designed to “ensure equitable access to vaccines, therapeutics, and diagnostics.” For PABS to operate successfully, it is necessary to implement large-scale genomic surveillance.
The network of collaborating laboratories and institutions will allow the flow of pathogen genomic data, using open-source databases and implementing the latest developments in bioinformatics.
WHO’s BioHub is envisioned as an operational component within the broader framework of the PABS system. Specifically, the PABS system is expected to govern and standardize how pathogens and their genetic data are shared globally, including through facilities like BioHub.
The first official WHO BioHub system was established at the military-controlled Swiss BSL-4 Spiez Laboratory.
Set-up after World War Two, this BSL-4 lab is located in the idyllic lakeside town of Spiez, 40 km from Switzerland’s capital city of Berne.
BioHub acts as a designated laboratory within the WHO Coordinated Laboratory Network conceived by the PABS system. While the above page is no longer on Spiez Laboratory’s website or found in English, this 2021 news release from the WHO is still available.
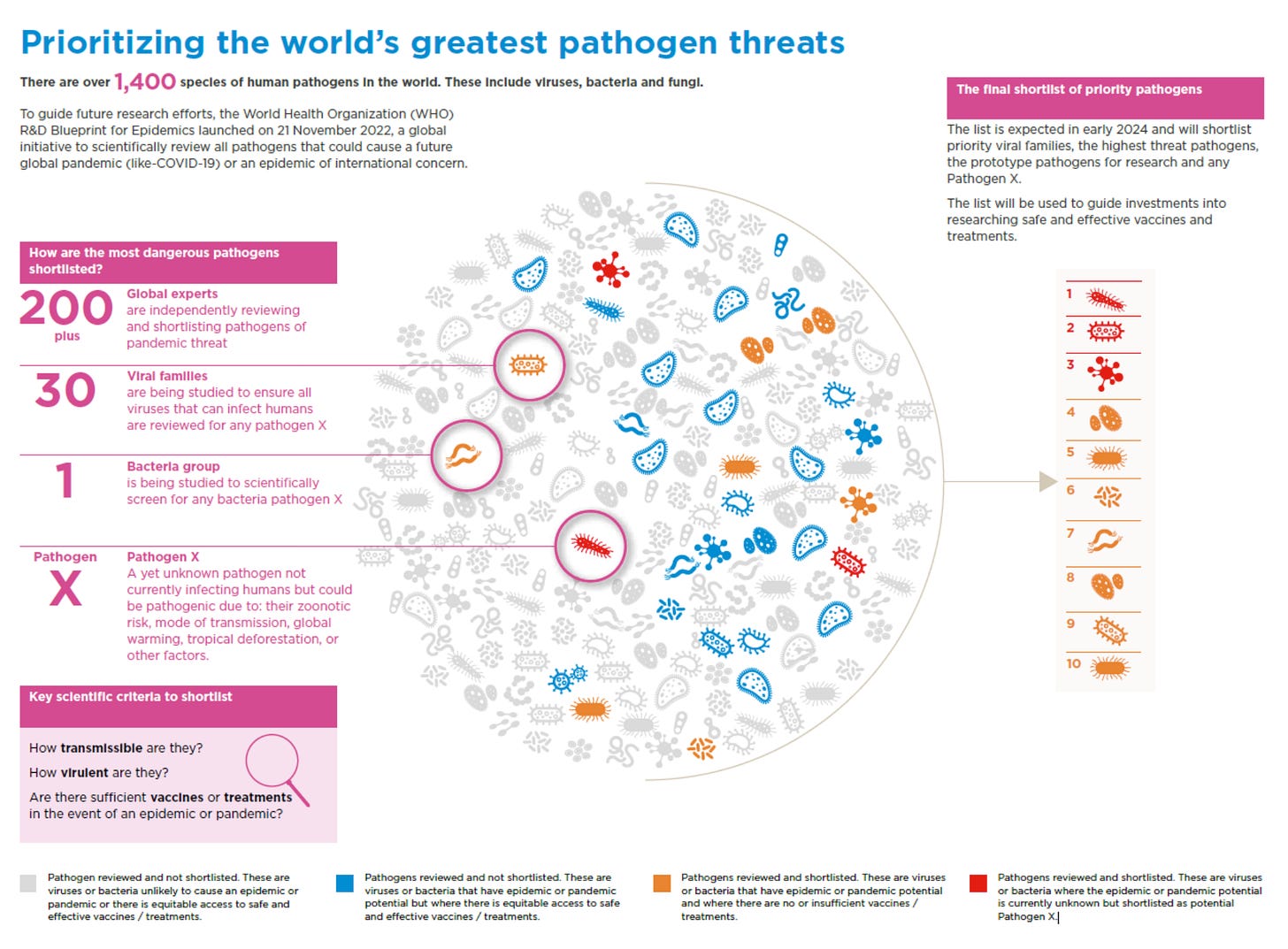
Getting back to the Maria Van Kerkhove’s presentation, she stressed the importance of this PABS system, highlighting the need to rapidly identify and catalogue priority pathogens.
At present, the non-exhaustive list of priority diseases is as follows:
COVID-19
Crimean-Congo hemorrhagic fever
Ebola and Marburg
Lassa fever
MERS/SARS
Nipah and henipaviral diseases
Rift Valley fever
Zika
Disease “X”.
The WHO’s latest approach, updated in 2024, now emphasizes a family-centric model rather than focusing solely on individual viruses. This shift recognizes that any viral family—even those currently considered low risk—could become a major threat due to genetic or environmental changes.
This approach significantly expands the portfolio of potentially pathogenic viruses, and the market for vaccines against them.

Remember that in the last sixty years, pandemics have only ever been viral, though there is a noteworthy list of bacterial pathogens considered high risk.
Below are a few documents worth sharing from Dr. Van Kerkhove’s powerpoint presentation:

“WHO has specific responsibilities and accountabilities for health emergency operations under the International Health Regulations (2005) (IHR) and the Inter-Agency Standing Committee (IASC), and as mandated in the WHO Thirteenth General Programme of Work 2019–2023. Further efforts are underway to strengthen the global architecture for health emergency preparedness, prevention, response and resilience (HEPR), at the national and international levels.”
– Page 10, Executive Summary
Dr. Van Kerkhove, like most speakers during the symposium, spoke out against President Trump’s order to cease US funding and withdraw its membership from the WHO. His decision is presented as a reckless move that will endanger many lives by gutting international health programs, notably for low- to middle-income countries.
In conclusion, Dr. Van Kerkhove insisted on the importance of working with WHO Member States and partners to strengthen governance and sustainable financing mechanisms for pandemic preparedness and response—which is estimated to cost around US$31 billion per year!
There was zero mention of any COVID-19 after-action review to assess the origins and the management of SARS-CoV-2 by the WHO.
During question time, Dr. Van Kerkhove shared that China continues to actively block efforts to investigate the Wuhan Institute of Virology’s involvement in the outbreak of SARS-CoV-2. She claimed that China’s interference in undertaking a thorough inquiry will make it impossible for the WHO to establish what actually happened and learn from the mistakes. She reminded everyone that the WHO can only report upon what it knows for a fact.
The audience is informed that an updated official WHO investigative report will pass by Dr. Van Kerkhove’s desk and should be released around the time of the 78th World Health Assembly in May—which has since come and passed with no word on this alleged document. The WHO’s most recent publicly-available report on COVID-19 origins is dated April 6, 2021.
The generally accepted source of the pandemic within the consensus-driven, mainstream scientific and academic community remains firmly zoonotic.
This is definitely the most comfortable explanation to avoid further political turmoil, scientific embarrassment and an even bigger dent in public trust.
Though the origins of the COVID-19 pandemic remain officially undetermined, a lab leak from China’s Wuhan Institute of Virology is deemed likely by the White House, FBI, CIA, former CDC director Robert Redfield, former UK Prime Minister Boris Johnson, the U.S. Energy Department and the U.S. House of Representatives’ Select Subcommittee on the Coronavirus Pandemic.
This year on April 1st—it was no April Fools Day joke—a French report was published by the prestigious Académie Nationale de Médecine, entitled From the Origin of SARS-CoV-2 to the Risks of Zoonoses and Dangerous Virus Manipulation. This analysis also leans towards a lab-leak origin, though no conclusive statement is made. It does however recommend that:
“Raising awareness among researchers and students of their scientific and ethical responsibilities regarding the risks of laboratory accidents/incidents is essential. The current context of technological developments in biology, including AI, can lead to serious consequences in the absence of control on their possible impact.”
On April 30, 2025, China's State Council Information Office launched a rebuttal suggesting that the USA should investigate themselves.
“Substantial evidence suggested the COVID-19 might have emerged in the United States earlier than its officially-claimed timeline, and earlier than the outbreak in China.”
Despite all these reports, during the symposium there was little to no mention of weaponised viruses and biosecurity risks emanating from BSL-4 laboratories.
Climate change and the destruction of ecosystems triggering animal spillover events was repeatedly cited during the Geneva symposium as being the likely cause of the next viral outbreak—which will probably be influenza.
Flashback to February 18, 2017, at the Munich Security Conference where billionaire Bill Gates warned of a highly pathogenic influenza virus.
“We also face a new threat that the next epidemic has a good chance of originating on a computer screen of a terrorist intent on using genetic engineering to create a synthetic version of the smallpox virus or a contagious and highly deadly strain of flu. So the point is that we ignore the strong link between health security and international security at our peril. Whether it occurs by the quirk of nature or at the hand of a terrorist, epidemiologists show through their models that a respiratory spread pathogen would kill more than 30 million people in less than a year.”
Back to the symposium and the next speaker….
Melissa Saville, MBBS / Chief Scientific Officer at PATH (ex-CEPI)
”The current landscape of new vaccines and vaccine innovation”
Dr. Saville praised the potential of computational antigen design for the conception of new vaccines and the promise “safe and effective” mRNA platforms, notably for gene delivery.
She commended the 350+ laboratories worldwide who used the SARS-CoV-2 sequence to make synthetic genes to enable rapid research and development in response to the COVID-19 pandemic.
While acknowledging that the mRNA COVID-19 vaccines do not prevent transmission and that they are linked to certain “safety events,” she insisted on the importance of exploring mucosal delivery systems to optimize protection. She referenced the Novo Nordisk Foundation’s work on developing promising mucosal vaccines against respiratory infections.
Did you know that the Novo Nordisk Foundation (NNF) is actually much wealthier than the Bill & Melinda Gates Foundation? Since the 1960s, the NNF has invested billions in scientific and medical research, though it has only recently got involved in vaccine development.
Dr. Saville alluded to the first mRNA vaccine developed in India by Gennova and the company’s investigation into creating self-amplifying vaccines (for Nipah virus) that replicate in cells, allowing for a reduction in the dose and lowered production costs.
Remember that as of April 2025, there is only one self-amplifying mRNA (sa-mRNA) vaccine on the market: KOSTAIVE® (ARCT-154) by CSL and Arcturus Therapeutics, approved for use against COVID-19 in the EU in February 2025, and licensed in Japan in November 2023.
While there is no sa-mRNA vaccine currently authorized in the US, on April 10th 2025, Arcturus Therapeutics received a “Fast Track Designation” for its STARR® (Self-Transcribing and Replicating RNA) sa-mRNA vaccine candidate, ARCT-2304, designed to protect humans against influenza A H5N1 (bird flu).
Dr. Saville spoke of trans-amplifying mRNA vaccines as being a promising platform currently under development. But what exactly is “trans-amplifying”?
Trans-amplifying RNA (taRNA) vaccines are a novel approach that splits the vaccine vector into two separate protein-coding RNA fragments: one encoding the antigen and another encoding the enzyme, replicase. This design allows the enzyme to amplify the antigen-encoding RNA within the cell. This strategy can significantly reduce the amount of RNA needed to elicit an immune response compared to traditional mRNA or sa-RNA vaccines.
Further reading on trans-amplifying vaccines in development:
A Trans-amplifying RNA Vaccine Strategy for Induction of Potent Protective Immunity (2020)
https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(19)30412-5A trans-amplifying RNA simplified to essential elements is highly replicative and robustly immunogenic in mice (2023)
https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(23)00019-9
Trans-Amplifying RNA: A Journey from Alphavirus Research to Future Vaccines (2024)
https://pmc.ncbi.nlm.nih.gov/articles/PMC11055088/New research to investigate next generation ‘trans-amplifying’ mRNA vaccines (2024)
https://cepi.net/new-research-investigate-next-generation-trans-amplifying-mrna-vaccines
Among other important medical countermeasures, Dr. Saville highlighted the importance of developing monoclonal antibodies (mAb) which she refers to as “quasi-vaccines” as part of a pandemic response “immunization strategy.”
Remember that the monoclonal antibody product Beyfortus®—nirsevimab, as passive immunization against respiratory syncytial virus (RSV)—is now included in the routine childhood immunization schedule in the US, Canada, Australia, the EU, (France, Spain, Germany), South Korea, Switzerland and the UK (for high-risk infants only). On May 30, 2025, the WHO officially recommended nirsevimab—produced by Sanofi in partnership with AstraZeneca/MedImmune, at the list price of US$519.75 per dose—to be given to all newborns to protect against RSV.
Dr. Saville concluded her presentation with highlighting the necessity of regional production with manufacturing facilities in Africa to achieve CEPI’s 100 Days Mission. She asserted that this will only be possible by collaborating with BARDA, PATH and Gavi as partners for coordinating and building local infrastructure.
Early steps in regional production have been achieved with BioNTech’s December 2024 inauguration of its first mRNA bioreactor factory in Africa, located in Kigali, Rwanda. Plans are in progress to open similar facilities in South Africa and Kenya.
On her very final note, Dr. Saville spoke out against the substantial funding cuts by both the US and the EU, forecasting negative impacts on global health projects in 50 countries, primarily affecting HIV programs.
Part 2 of this article will provide details on some of the other key speakers during this April 2025 symposium and the main points worth sharing.
If there is a specific presentation from the program that you are particularly interested in knowing more about, please let me know by leaving a comment below.